Nutrients in the Compost Pile
by Steve Solomon
Some types of leaves rot much faster on the forest floor than others. Analyzing why this happens reveals a great deal about how to make compost piles decompose more effectively.
Leaves from leguminous (in the same botanical family as beans and peas) trees such as acacia, carob, and alder usually become humus within a year. So do some others like ash, cherry, and elm. More resistant types take two years; these include oak, birch, beech, and maple. Poplar leaves, and pine, Douglas fir, and larch needles are very slow to decompose and may take three years or longer. Some of these differences are due to variations in lignin content which is highly resistant to decomposition, but speed of decomposition is mainly influenced by the amount of protein and mineral nutrients contained in the leaf.
Plants are composed mainly of carbohydrates like cellulose, sugar, and lignin. The element carbon is by far the greater part of carbohydrates [carbo(n)hydr(ogen)ates] by weight. Plants can readily manufacture carbohydrates in large quantities because carbon and hydrogen are derived from air (C02) and water (H2O), both substances being available to plants in almost unlimited quantities.
Sugar, manufactured by photosynthesis, is the simplest and most vital carbohydrate. Sugar is "burned" in all plant cells as the primary fuel powering all living activities. Extra sugar can be more compactly stored after being converted into starches, which are long strings of sugar molecules linked together. Plants often have starch-filled stems, roots, or tubers; they also make enzymes capable of quickly converting this starch back into sugar upon demand. We homebrewers and bakers make practical use of a similar enzyme process to change starches stored in grains back to sugar that yeasts can change into alcohol.
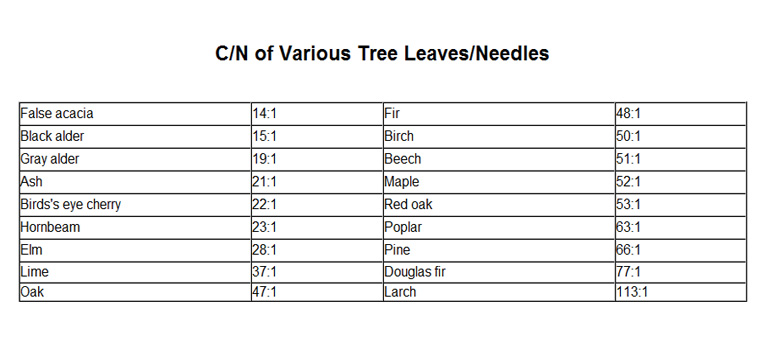
The protein content of tree leaves is very similar to their ratio of carbon (C) compared to nitrogen (N)
Sometimes plants store food in the form of oil, the most concentrated biological energy source. Oil is also constructed from sugar and is usually found in seeds. Plants also build structural materials like stem, cell walls, and other woody parts from sugars converted into cellulose, a substance similar to starch. Very strong structures are constructed with lignins, a material like cellulose but much more durable. Cellulose and lignins are permanent. They cannot be converted back into sugar by plant enzymes. Nor can most animals or bacteria digest them.
Certain fungi can digest cellulose and lignin, as can the symbiotic bacteria inhabiting a cow's rumen. In this respect the cow is a very clever animal running a cellulose digestion factory in the first and largest of its several stomachs. There, it cultures bacteria that eat cellulose; then the cow digests the bacteria as they pass out of one stomach and into another.
Plants also construct proteins, the vital stuff of life itself. Proteins are mainly found in those parts of the plant involved with reproduction and photosynthesis. Protein molecules differ from starches and sugars in that they are larger and amazingly more complex. Most significantly, while carbohydrates are mainly carbon and hydrogen, proteins contain large amounts of nitrogen and numerous other mineral nutrients.
Proteins are scarce in nature. Plants can make them only in proportion to the amount of the nutrient, nitrogen, that they take up from the soil. Most soils are very poorly endowed with nitrogen. If nitrate-poor, nutrient-poor soil is well-watered there may be lush vegetation but the plants will contain little protein and can support few animals. But where there are high levels of nutrients in the soil there will be large numbers of animals, even if the land is poorly watered and grows only scrubby grasses—verdant forests usually feed only a few shy deer while the short grass semi-desert prairies once supported huge herds of grazing animals.
Ironically, just as it is with carbon, there is no absolute shortage of nitrogen on Earth. The atmosphere is nearly 80 percent nitrogen. But in the form of gas, atmospheric nitrogen is completely useless to plants or animals. It must first be combined chemically into forms plants can use, such as nitrate (NO3) or ammonia (NH3). These chemicals are referred to as "fixed nitrogen."
Nitrogen gas strongly resists combining with other elements. Chemical factories fix nitrogen only at very high temperatures and pressures and in the presence of exotic catalysts like platinum or by exposing nitrogen gas to powerful electric sparks. Lightning flashes can similarly fix small amounts of nitrogen that fall to earth dissolved in rain.
And certain soil-dwelling microorganisms are able to fix atmospheric nitrogen. But these are abundant only where the earth is rich in humus and minerals, especially calcium. So in a soil body where large quantities of fixed nitrogen are naturally present, the soil will also be well-endowed with a good supply of mineral nutrients.
Most of the world's supply of combined nitrogen is biologically fixed at normal temperatures and standard atmospheric pressure by soil microorganisms. We call the ones that live freely in soil "azobacteria" and the ones that associate themselves with the roots of legumes "rhizobia." Blue-green algae of the type that thrive in rice paddies also manufacture nitrate nitrogen. We really don't know how bacteria accomplish this but the nitrogen they "fix" is the basis of most proteins on earth.
All microorganisms, including nitrogen-fixing bacteria, build their bodies from the very same elements that plants use for growth. Where these mineral elements are abundant in soil, the entire soil body is more alive and carries much more biomass at all levels from bacteria through insects, plants, and even mammals.
Should any of these vital nutrient substances be in short supply, all biomass and plant growth will decrease to the level permitted by the amount available, even though there is an overabundance of all the rest. The name for this phenomena is the "Law of Limiting Factors." The concept of limits was first formulated by a scientist, Justus von Liebig, in the middle of the last century. Although Liebig's name is not popular with organic gardeners and farmers because misconceptions of his ideas have led to the widespread use of chemical fertilizers, Liebig's theory of limits is still good science.
Liebig suggested imagining a barrel being filled with water as a metaphor for plant growth: the amount of water held in the barrel being the amount of growth. Each stave represents one of the factors or requirements plants need in order to grow such as light, water, oxygen, nitrogen, phosphorus, copper, boron, etc. Lowering any one stave of the barrel, no matter which one, lessens the amount of water that can be held and thus growth is reduced to the level of the most limited growth factor.
For example, one essential plant protein is called chlorophyll, the green pigment found in leaves that makes sugar through photosynthesis. Chlorophyll is a protein containing significant amounts of magnesium. Obviously, the plant's ability to grow is limited by its ability to find enough fixed nitrogen and also magnesium to make this protein.
Animals of all sizes from elephants to single cell microorganisms are primarily composed of protein. But the greatest portion of plant material is not protein, it is carbohydrates in one form or another. Eating enough carbohydrates to supply their energy requirements is rarely the survival problem faced by animals; finding enough protein (and other vital nutrients) in their food supply to grow and reproduce is what limits their population. The numbers and health of grazing animals is limited by the protein and other nutrient content of the grasses they are eating, similarly the numbers and health of primary decomposers living on the forest floor is limited by the nutrient content of their food. And so is the rate of decomposition. And so too is this true in the compost pile.
The protein content of vegetation is very similar to its ratio of carbon (C) compared to nitrogen (N). Quick laboratory analysis of protein content is not done by measuring actual protein itself but by measuring the amount of combined nitrogen the protein gives off while decomposing. Acacia, alder, and leaves of other proteinaceous legumes such as locust, mesquite, scotch broom, vetch, alfalfa, beans, and peas have low C/N ratios because legume roots uniquely can shelter clusters of nitrogen-fixing rhizobia. These microorganisms can supply all the nitrate nitrogen fast-growing legumes can use if the soil is also well endowed with other mineral nutrients rhizobia need, especially calcium and phosphorus. Most other plant families are entirely dependent on nitrate supplies presented to them by the soil. Consequently, those regions or locations with soils deficient in mineral nutrients tend to grow coniferous forests while richer soils support forests with more protein in their leaves. There may also be climatic conditions that favor conifers over deciduous trees, regardless of soil fertility.
It is generally true that organic matter with a high ratio of carbon to nitrogen also will have a high ratio of carbon to other minerals. And low C/N materials will contain much larger amounts of other vital mineral nutrients. When we make compost from a wide variety of materials there are probably enough quantity and variety of nutrients in the plant residues to form large populations of humus-forming soil animals and microorganisms. However, when making compost primarily with high C/N stuff we need to blend in other substances containing sufficient fixed nitrogen and other vital nutrient minerals. Otherwise, the decomposition process will take a very long time because large numbers of decomposing organisms will not be able to develop.
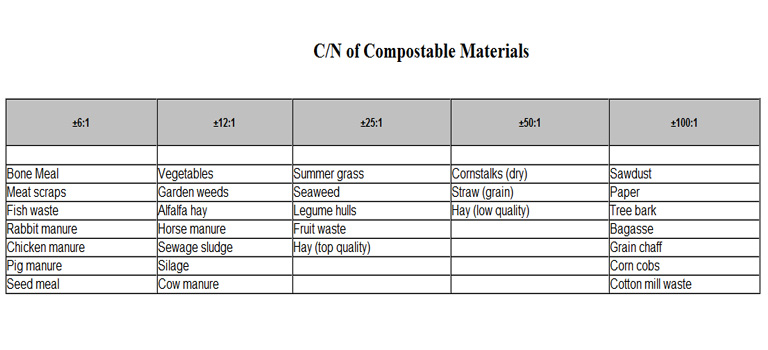
The lists in this table of carbon/nitrogen ratios are broken out as general ranges of C/N. It has long been an unintelligent practice of garden-level books to state "precise" C/N ratios for materials. One substance will be "23:1" while another will be "25:1." Such pseudoscience is not only inaccurate but it leads readers into similar misunderstandings about other such lists, like nitrogen contents, or composition breakdowns of organic manures, or other organic soil amendments. Especially misleading are those tables in the back of many health and nutrition books spelling out the "exact" nutrient contents of foods. There is an old saying about this: 'There are lies, then there are damned lies, and then, there are statistics. The worse lies of all can be statistics.'
The composition of plant materials is very dependent on the level and nature of the soil fertility that produced them. The nutrition present in two plants of the same species, even in two samples of the exact same variety of vegetable raised from the same packet of seed can vary enormously depending on where the plants were grown. William Albrecht, chairman of the Soil Department at the University of Missouri during the 1930s, was, to the best of my knowledge, the first mainstream scientist to thoroughly explore the differences in the nutritional qualities of plants and to identify specific aspects of soil fertility as the reason why one plant can be much more nutritious than another and why animals can be so much healthier on one farm compared to another. By implication, Albrecht also meant to show the reason why one nation of people can be much less healthy than another. Because his holistic outlook ran counter to powerful vested interests of his era, Albrecht was professionally scorned and ultimately left the university community, spending the rest of his life educating the general public, especially farmers and health care professionals.
Summarized in one paragraph, Albrecht showed that within a single species or variety, plant protein levels vary 25 percent or more depending on soil fertility, while a plant's content of vital nutrients like calcium, magnesium, and phosphorus can simultaneously move up or down as much as 300 percent, usually corresponding to similar changes in its protein level. Albrecht also discovered how to manage soil in order to produce highly nutritious food. Chapter Eight has a lot more praise for Dr. Albrecht. There I explore this interesting aspect of gardening in more detail because how we make and use organic matter has a great deal to do with the resulting nutritional quality of the food we grow.
Imagine trying to make compost from deficient materials such as a heap of pure, moist sawdust. What happens? Very little and very, very slowly. Trees locate most of their nutrient accumulation in their leaves to make protein for photosynthesis. A small amount goes into making bark. Wood itself is virtually pure cellulose, derived from air and water. If, when we farmed trees, we removed only the wood and left the leaves and bark on the site, we would be removing next to nothing from the soil. If the sawdust comes from a lumber mill, as opposed to a cabinet shop, it may also contain some bark and consequently small amounts of other essential nutrients.
Thoroughly moistened and heaped up, a sawdust pile would not heat up, only a few primary decomposers would take up residence. A person could wait five years for compost to form from pure moist sawdust and still not much would happen. Perhaps that's why the words "compost" and "compot" as the British mean it, are connected. In England, a compot is a slightly fermented mixture of many things like fruits. If we mixed the sawdust with other materials having a very low C/N, then it would decompose, along with the other items.





